Here is a conversation from the comments of my last post on the FDA with fellow progress blogger Alex Telford that follows a pattern common to many of my conversations about the FDA:
Alex: Most drugs that go into clinical trials (90%) are less effective or safe than existing options. If you release everything onto the market you'll get many times more drugs that are net toxic (biologically or financially) than the good drugs you'd get faster. You will almost surely do net harm.
Max: Companies don't want to release products that are worse than their competitors.
Companies test lots of cars or computers or ovens which are less effective or safe than existing options but they only release the ones that are competitive. This isn't because most consumers could tell whether their car was less efficient or that their computer is less secure, and it's not because making a less efficient car or less secure computer is against the law.
Pharmaceutical companies won't go and release hundreds of dud or dangerous drugs just because they can. That would ruin their brand and shut down their business. They have to sell products that people want.
Alex: Consumer products like ovens and cars aren't comparable to drugs. The former are engineered products that can be tested according to defined performance and safety standards before they are sold to the public. The characteristics of drugs are more discovered than engineered. You can't determine their performance characteristics in a lab, they can only be determined through human testing (currently).
Alex claims that without the FDA, pharmaceutical companies would release lots of bunk drugs. I respond that we don’t see this behavior in other markets. Car companies or computer manufacturers could release cheaply made, low quality products for high prices and consumers might have a tough time noticing the difference for a while. But they don’t do this, they always try to release high quality products at competitive prices.
Alex responds, fairly, that car or computer markets aren’t comparable to drug markets. Pharmaceuticals have stickier information problems. They are difficult for consumers to evaluate and, as Alex points out, usually require human testing.
This is usually where the conversation ends. I think that consumer product markets are informative for what free-market pharmaceuticals would look like, Alex (and lots of other reasonable people) don’t and it is difficult to convince each other otherwise.
But there’s a much better non-FDA counterfactual for pharmaceutical markets than consumer tech: surgery.
The FDA does not have jurisdiction over surgical practice and there is no other similar legal requirement for safety or efficacy testing of new surgical procedures. The FDA does regulate medical devices like the da Vinci surgical robot but once they are approved surgeons can use them in new ways without consulting the FDA or any other government authority.
In addition to this lack of regulation, surgery is beset with even thornier information problems than pharmaceuticals. Evaluating the quality of surgery as a customer is difficult. You’re literally unconscious as they provide the service and retrospective observation of quality is usually not possible for a layman. Assessing quality is difficult even for a regulator, however. So much of surgery hinges on the skill of a particular surgeon and varies within surgeons day to day or before and after lunch.
Running an RCT on a surgical technique is therefore difficult. Standardizing treatment as much as in pharmaceutical trials is basically impossible. It also isn’t clear what a surgical placebo should be. Do just put them under anesthetic for a few hours? Or do you cut people open and stitch them up without doing anything else? So surgical RCTs are rare and small when they happen.
Despite extreme information problems and a complete absence of federal oversight, surgery seems to work well. Compared to similar patients on the waiting list, 2.3 million life years were saved by organ transplants over 25 years. The WHO claims that “surgical interventions account for 13% of the world’s total disability-adjusted life years.” Coronary artery surgery extends lifespan by several years for $2300 a year. Cataract surgery and LASIK can massively improve quality of life for a few thousand dollars.
Surgery is also improving over time. Pancreaticoduodenectomy, the surgical treatment for pancreatic cancer, began in the late 19th century as a near death sentence, although to be fair so was pancreatic cancer. For several decades surgeons were unsure whether it was even possible to survive anything except partial versions of the procedure. They experimented on dogs, cadavers, and consenting terminal patients and improved the procedure. Today, the procedure is safe and a standard treatment for pancreatic cancer. From 2006-2012 the mortality rate halved from 2.9% to 1.5%.
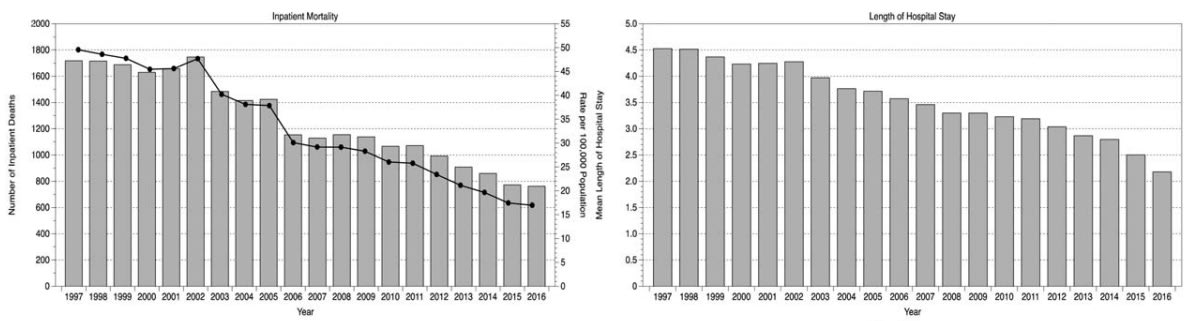
On a larger scale, among all emergency general surgery episodes in Scotland from 1997 to 2016, inpatient mortality mortality rates declined by more than three times and hospital stay lengths were cut in half, probably due to the rise of minimally invasive and robotic surgery tools.
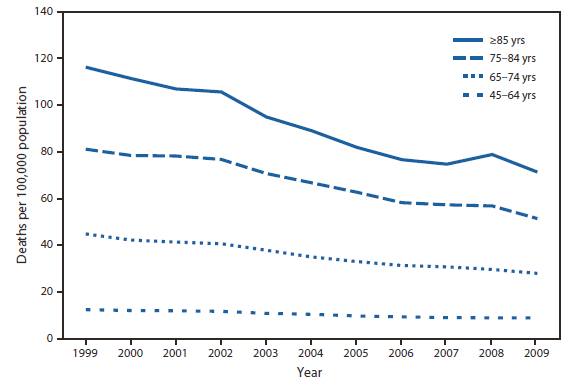
In the US, the death rate from medical and surgical care complications declined by 39% from 1999 to 2009.
Operative mortality for eight different cancer and cardiovascular operations from 1999 through 2008 declined with the lowest decline being 8% and the highest at 36%.
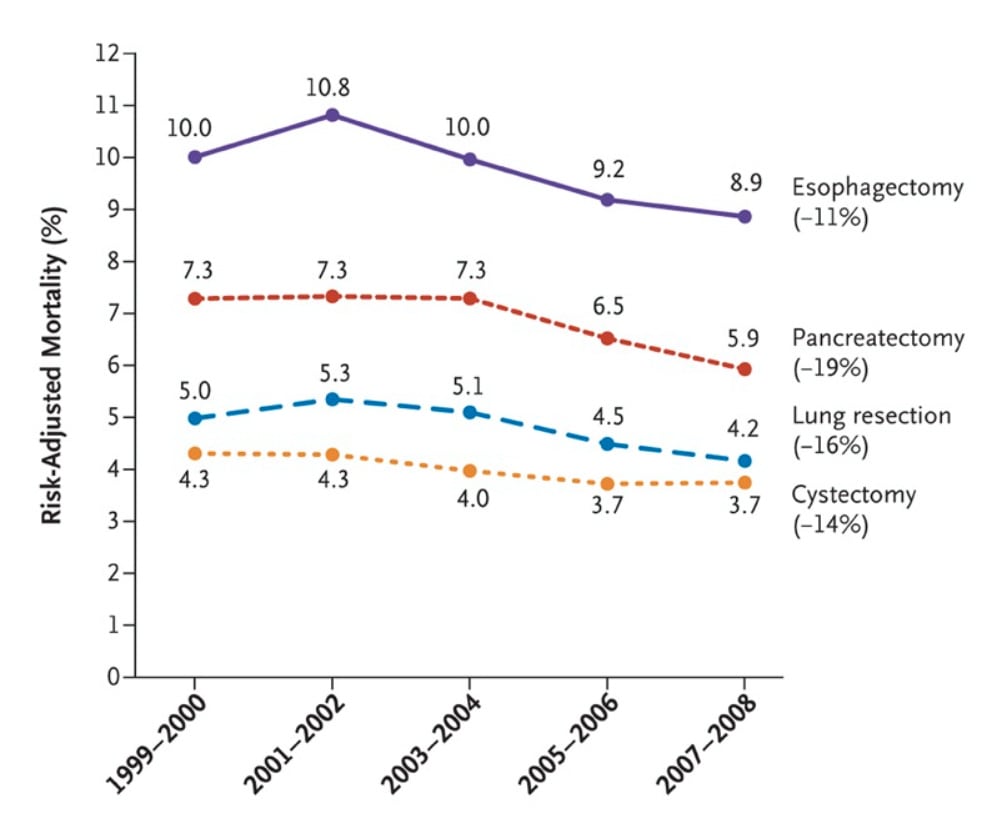
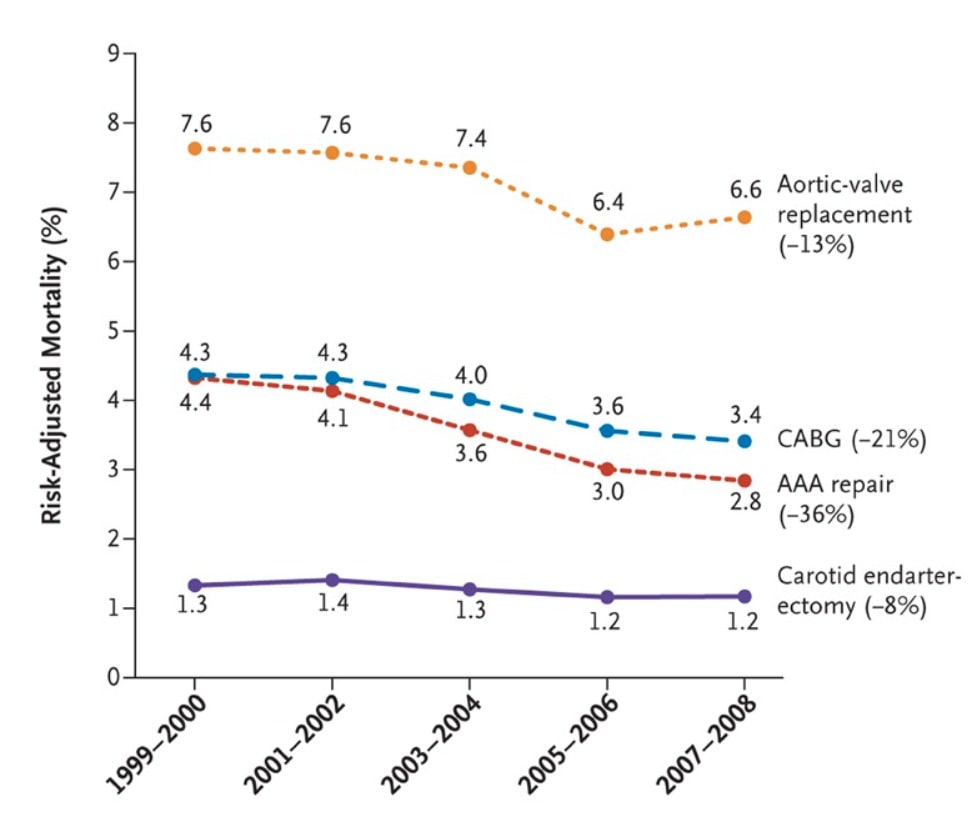
These are common surgeries with hundreds of thousands of operations each year.
There are problems with surgery. There are unnecessary deaths and ineffective procedures. There are bad actors and high prices. But surgery is not the vision of snake oil and certain death that is painted by many FDA defenders when you ask them to imagine a world without the FDA. The problems faced by surgery are matched and sometimes exceeded by problems faced by pharmaceuticals.
There are also non-governmental mechanism which constrain surgeons and protect consumers. Competition, insurance companies, hospital lawyers and ethics boards. But this strengthens the point. An FDA model is not the only way to promote consumer protection and solve the information problems presented by medicine and surgery.
So we don’t need to argue about the validity of consumer tech or cars as a non-FDA counterfactual for pharmaceuticals. Surgery has at least all of the market challenges that harry pharmaceuticals and it doesn’t have an FDA. Yet, it still muddles through. This is what we should expect pharmaceuticals to look like if we abolished the FDA. We would have a well-functioning, self-improving market for medical treatments without billion-dollar fifteen-year waits for any new drug that people want to try.

I wrote my rebuttal to this article here.
Great discussion and examples. I often struggle to explain why bad business fails without regulators but is propped up by them far too often. This was a helpful article on the topic and your ideas are valuable. Keep writing!